A male patient in his 60s has a history of Parkinson’s disease. He also had a history of esophageal cancer, hyperlipidemia, gastroesophageal reflux disease, asthma, arthritis and anxiety.
Presentation and Examination
The patient was preparing to get deep brain stimulation (DBS) to treat his Parkinson’s disease. On his pre-DBS MRI study, he was incidentally found to have a 5-6 mm left middle cerebral artery bifurcation aneurysm. Options discussed included observation, surgical clipping and endovascular treatment. His desire was to have it treated endovascularly. An extensive discussion was had regarding the timing of the aneurysm treatment and the DBS procedure. Due to the relatively wide-necked morphology of the aneurysm, there was the possibility that he would require dual- antiplatelet therapy for stent placement, which could complicate a subsequent DBS surgery. I therefore recommended that he have the DBS surgery as planned, followed by endovascular repair of the aneurysm.
Treatment
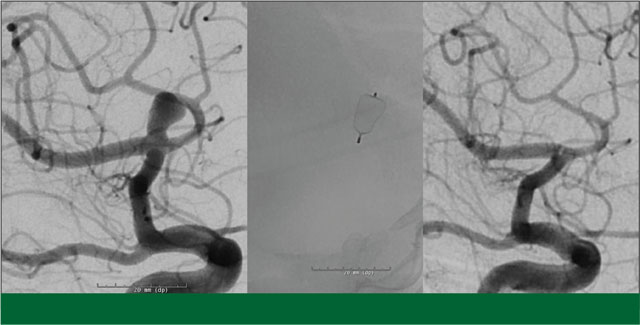
The patient underwent an uncomplicated DBS surgery by Sepehr Sani, MD, at Rush University Medical Center. Six weeks later he returned for treatment of his aneurysm. This was done endovascularly through a needle puncture in the groin. A small catheter was inserted into the femoral artery and advanced into the left internal carotid artery. An angiogram confirmed that the aneurysm was a reasonable candidate for endovascular treatment. In my opinion, the wide-necked geometry meant that his best options were stent-assisted coil embolization, or placement of a WEB device. I felt that if we utilized the WEB, it would eliminate the need for long-term antiplatelet therapy which is typically used with stent placement. Using continuous fluoroscopic imaging, a single WEB device was deployed into the aneurysm, immediately minimizing blood flow inside the aneurysm.
The patient tolerated the procedure well and was discharged the following day. While he had been started on aspirin and plavix prior to the treatment in anticipation of a possible stent, we were able to discontinue the plavix and aspirin following the procedure.
Facts about the Woven EndoBridge (WEB) Aneurysm Embolization System (from the Food and Drug Administration)
What is it? The Woven EndoBridge (WEB) Aneurysm Embolization System is a permanent nitinol (nickel titanium) self-expanding mesh ball implant for the treatment of wide-neck intracranial aneurysms located at or near branching areas of arteries in the brain. The device system consists of the implant, the delivery wire, and the controller that is used to detach the device from the delivery wire.
How does it work? The WEB Aneurysm Embolization System is introduced into the blood vessels in a small artery either in the groin or wrist. The WEB implant is then detached from the delivery system and placed into the intracranial aneurysm. The WEB implant is designed to disrupt blood flow entering the aneurysm and help promote clotting (thrombosis).
When is it used? The WEB Aneurysm Embolization System is indicated for use in the following arteries located in the brain (middle cerebral artery (MCA) bifurcation, internal carotid artery (ICA) terminus, anterior communicating artery (AComm) complex, or basilar artery apex) for the treatment of adult patients with saccular, wide neck, bifurcation intracranial aneurysms with dome diameter from 3 mm to 10 mm and either neck size 4 mm or greater or the dome-to-neck ratio is greater than 1 and less than 2.
What will it accomplish? The WEB Aneurysm Embolization System is designed to promote aneurysmal occlusion.
When should it not be used? The WEB Aneurysm Embolization System should not be used in patients with known:
- Active bacterial infection that may interfere with or negatively affect the implantation procedure
- Hypersensitivity to nickel
Outcome
The patient did very well after his WEB placement, with no related neurological sequelae. A follow up angiogram performed 18 months after his procedure showed complete cure of the aneurysm. In addition, he feels his Parkinson’s tremor is substantially improved following DBS treatment.
Analysis
As a minimally invasive option for treating wide- neck bifurcation aneurysms in certain areas of the brain, the WEB Aneurysm Embolization System is an option for the treatment of cerebral aneurysms that has the potential to substantially impact the delivery of care to patients with these lesions. Due to its ability to be used for wide-necked aneurysms, it may allow some aneurysms that previously
were felt to require craniotomy and clipping to now be treated endovascularly, and avoids the need for long-term anti-platelet therapy that is required with stent placement. This can result in improved safety and shorter recovery times. When used to treat an unruptured aneurysm, patients can typically go home the following day after the device is implanted. By fixing the aneurysm before it ruptures, the threat of an aneurysm bursting and the patient dying from it essentially goes away.
The WEB system has been shown to be highly effective and safe. According to the FDA, in a clinical study of 150 subjects in which treatment with the device was attempted, 54.77% of subjects met the primary effectiveness endpoint and achieved a successful treatment (complete occlusion) of their intracranial aneurysm within 1-year post-procedure without re-treatment, recurrent subarachnoid hemorrhage or clinically significant parent artery stenosis.
An estimated 6 million people in the United States have an unruptured brain aneurysm, with 35% of them being wide-necked bifurcation aneurysms. The WEB system may be an option for thousands of these patients, providing a safe, effective alternative to existing treatment options, including potentially avoiding the need for craniotomy and clipping in some patients.

